Updated/reviewed by the author, July 2017.
Serous PED
Authors:
Ugo Introini, MD, Giuseppe Casalino, MD
Department of Ophthalmology and University Vita-Salute
Scientific Institute San Raffaele Milano, Italy
Chair: Prof Francesco Bandello
Serous PED in AMD
Retinal pigment epithelial detachment (PED) is part of age-related macular degeneration (AMD) clinical spectrum.
However, different types of PED have been reported in the literature and they have been either related or not with AMD.
Serous PED is defined as an area of sharply demarcated, dome-shaped serous elevation of the retinal pigment epithelium (RPE). The histopathology of serous PED is consistent with the detachment of the RPE basement membrane, along with the overlying RPE from the remaining Bruch’s membrane due to accumulation of fluid(1).
The presence of this lesion is a negative prognostic factor for AMD in terms of visual acuity outcome.
While no definite therapeutic indications have been set so far, early detection of serous PED is important for the prognosis and the management of patients with AMD.
In AMD, serous PED can be either associated or not with choroidal new vessels (choroidal neovascularization – CNV). However, the vascularised type is by far the most observed.
Several theories regarding the relationship between serous PED and CNV have been proposed.
To explain its pathogenesis, Gass theorized the growth of new vessels from the choroid (Type 1 neovascularization (NV)) inside the Bruch’s membrane thickness, that actively leak, increasing the hydrostatic pressure and causing RPE detachment among the less adherent layers(2).
This concept has been later supported by the evidence that the development of CNV comes with inflammatory mechanisms that add more damage to Bruch’s membrane, supporting RPE separation from the inner collagenous layer(3-5).
When the growth of new vessels starts from the inner retina, more recently described as Type 3 NV and also known as retinal angiomatous proliferation (RAP), it has been hypothesized that the serous PED formation, which is very frequently associated, can be related to RPE invasion by the neovascular complex6-8).
By contrast, other authors observed that the presence of PED can represent a pre-existing condition that can promote CNV growth through a further Bruch’s membrane damage, expression of the same ongoing disease(9,10).
Although the pathogenesis of the PED is not completely understood, from these studies the formation of NV seems to be a pivotal moment.
At fundus examination, serous PED appears as a round or oval, distinct dome-shaped area of regular detachment of the RPE and the overlying neurosensory retina, with yellow to orange color and smooth surface. Margins are typically sharply demarcated; and focal RPE atrophy and pigment figures are frequently observed(9,11).
However, the concomitant presence of NV can generate a variety of associated ophthalmoscopic aspects, such as hemorrhagic and exudative components, areas of irregular elevation of the RPE and serous detachment of the surrounding neuroretina.
Presentation of Type 1 NV located at the margin of the PED may vary, usually resulting in a reniform or notched aspect, or a flat-sided RPE detachment(12).
Serous PED may be imaged by fluorescein angiography (FA), indocyanine green angiography (ICGA) and optical coherence tomography (OCT).
FA represents, however, the gold standard for diagnosis of serous PED.
Examined by FA, serous PED classically shows an early uniform hyperfluorescence of the entire lesion, slightly delayed compared to the background fluorescence, that progressively increases in brightness as the examination progresses (pooling).
Serous PED hyperfluorescence typically does not change in size or shape during the angiographic phases.
FA can also demonstrate the presence of NV, usually associated to serous PED as Type 1 NV, like areas of indistinct late subretinal staining, more evident when located at the margin of the RPE detachment or corresponding to the “notch”(11).
The presence of NV can be also deducted by the presence of an hemorrhagic component of the PED, the dark meniscus described by Gass(12).
However, a more precise localization of the neovascular component can be obtained with digital ICGA. Indocyanine green molecule has biophysical properties that, unlike fluorescein, make it useful to enhance vessels anatomy through RPE, blood and turbid exudation.
In detail, ICGA enables to better delineate the presence and the type of new vessels associated with a serous PED, and for this reason is considered a fundamental tool in the management of this disease(13-15).
On ICGA, serous PED appears as an hypofluorescent lesion, with sharply delineated margins, that remains constantly hypofluorescent during all the phases of the examination(16).
When the new vessels are not present, no signs of localized hyperfluorescent areas are detectable; the outline of the PED is sharply round and it is therefore considered a pure serous PED.
In AMD patients, Yannuzzi found an incidence of 4% of non-vascularized PED among serous PED(15).
When the neovascular component is present, it has been suggested the term vascularized PED(15),.which accounts to approximately 24% of newly diagnosed exudative AMD(17).
New vessels associated with serous PED are represented in different subtypes.
High-speed videoangiography with scanning laser ophthalmoscope appears as a precious tool that allows the ophthalmologist to identify the new vessel pattern and their angiographic behaviour(18).
Recognize the different types of NV, by distinguishing angiographic findings, is mandatory for the distinct natural course, visual prognosis and different response to the treatment of the three main kinds of new vessels associated to serous PED in AMD.
The most common type of new vessels associated with serous PED are those occurring from the choroid beneath the RPE monolayer(15-,17).
These new vessels have been recently classified as Type 1 NV and are by far the most common type of NV in AMD(19)(Figure 1).
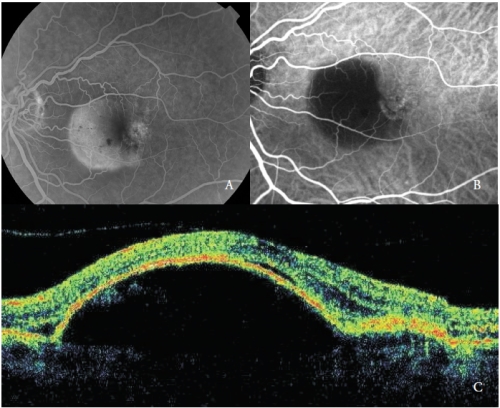
Figure 1 - Vascularized PED with Type 1 NV. (A) FA, (B)ICGA and (C) OCT.
In the early phases, ICGA shows the NV feeder artery that arises from the choroidal circulation, and subsequently the draining venule.
At the same time the capillary network of the neovascular membrane can be detected.
Unlike fluorescein, indocyanine green leaks slightly and the NV hyperfluorescence is usually minimal, with the exception of some cases that show an intense leakage, considered as very active new vessels.
Frequently, in the late phases, a well-defined area of mild hyperfluorescence corresponding to the NV network can be appreciable.
The second type of new vessels complicating serous PED are the RAP(7,20-22), also referred as Type 3 NV(19).
These vascular lesions, as reported by various authors, may involve the outer retina and the RPE, through a progression that has been hypothesized to originate from the retinal circulation and/or choroid.
ICGA typically shows the presence of a “hot-spot”, due to the early hyperfluorescence of the intraretinal neovascular complex, that increases during the angiography, with an intense leakage in the late phases.
Its brightness is enhanced by the surrounding hypofluorescence of the underlying PED (Figure 2).
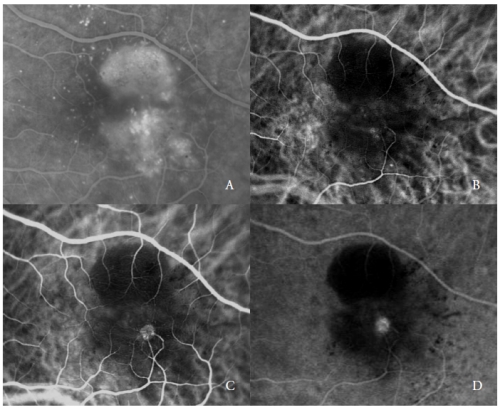
Figure 2 - Vascularized PED with Type 3 NV (RAP). FA (A) and ICGA early (B) and late phases (C and D).
In the late stages of the disease, the choroidal neovascular complex is typically connected with one or more retinal vessels which appear tortuous and dilated(7,22,23).
The Type 3 NV may be single or multiple, its origin is typically extrafoveal, and an intraretinal hemorrhage in correspondence of the neovascular lesion is frequently observed(20).
The third type of new vessels associated with serous PED in AMD is consistent with polypoidal choroidal vasculopathy (PCV)(24).
PCV is a peculiar form of CNV, characterized by the presence of orange, aneurismal, polyp-like round dilatations at the border of a branching vascular network of choroidal origin.
Although PCV affects more frequently middle-age black and asian populations, its clinical spectrum is expanded to whites, where it has been found to be present in 8-13% of patients with concomitant AMD lesions.
In these cases, when the manifestations attributable to both PCV and AMD are present, some authors consider PCV as a subtype of CNV in AMD(24,25).
Hemorrhagic manifestation is common in patients with PCV.
Serous PED associated with PCV shows frequently a blood level in the lower portion of the detachment.
ICGA is the state-of-the-art examination to distinguish the typical features of the two vascular components.
The vascular network is characterized by the presence of one or more aneurismal lesions that show a bright fluorescence since the early phases, followed in the late phases by a clearing of the dye, called “wash-out”, typical of this disease (Figure 3).
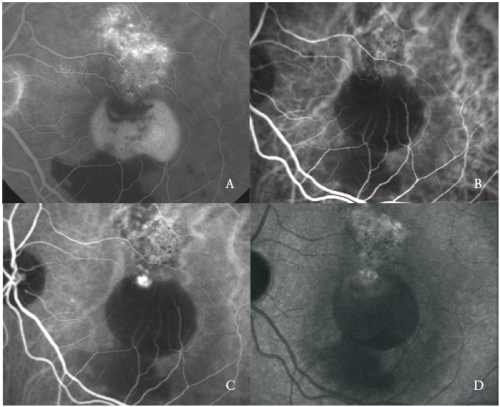
Figure 3 - Vascularized PED with PCV: FA (A) and early (B), mid (C) and late (D) phases of ICGA.
Nevertheless, some polyp-like structures can actively leak showing late staining of their walls and surrounding exudation.
The polypoidal lesions are usually located at the margin of the serous PED(26).
Recognition of these lesions is critical because of their different clinical course, prognosis and treatment response as compared to the other neovascular AMD subtypes.
OCT provides images that allow an exact correlation with the angiographic findings.
In cross-sectional OCT scans, serous PED appears as an optically empty dome-shaped elevation of the external high reflective band – the RPE, that steeply detaches from Bruch’s membrane(26).
The overlying retina, usually adherent to the bullous PED, at the margins of the lesion, can be slightly detached from the underlying RPE.
More additional information can be provided by OCT in vascularized PED(28).
The tomographic sections, guided by FA and ICGA in the area corresponding to the CNV, show a smoother elevation of the RPE, continuous with the serous detachment, with a deeper backscattering, due to the presence of the fibrovascular tissue.
Hyporeflective areas of homogeneous optically empty spaces referable to fluid accumulation are frequently present in the intraretinal and subretinal spaces(29).
Intraretinal optically empty spaces are more pronounced when the serous PED is associated with a Type 3 NV, especially with cystic shape (Figure 4).
By positioning the scan line corresponding to the “hot-spot”, the neovascular abnormality is represented as a dense or hyperreflective pre-epithelial zone in the inner retinal layers, where the outer hyperreflectant layers are no longer detectable(30).
The RPE close to that lesion shows frequently effractions or interruptions in its hyperreflective layer(31).
The retinal topographic measurement sustains an increased retinal thickness.
In eyes with serous PED and PCV, the polypoidal lesions show a sharp protrusion of RPE, similar to the PED but steeply sloped.
The polyps cavity, usually optically empty, is contiguous to irregular RPE elevation, expression of the occult neovascular component of the lesion(32,33).
Subretinal and intraretinal fluid, observed as hypofluorescent optical empty areas, are related to the PCV activity (Figure 4-B).
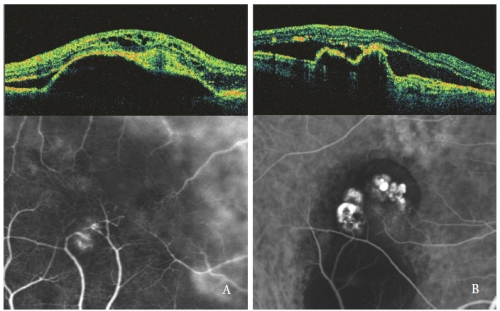
Figure 4 - Type 3 NV (RAP) (left) and PCV (right): OCT and ICGA patterns.
The recent introduction of OCT angiography (OCT-A) has made it possible to image perfusion of the different retinal layers, without injection of the dye and by utilizing endoluminal flow as intrinsic contrast.
Figure 5 provides an example of how OCT-A image the choroidal network in a case of PCV.
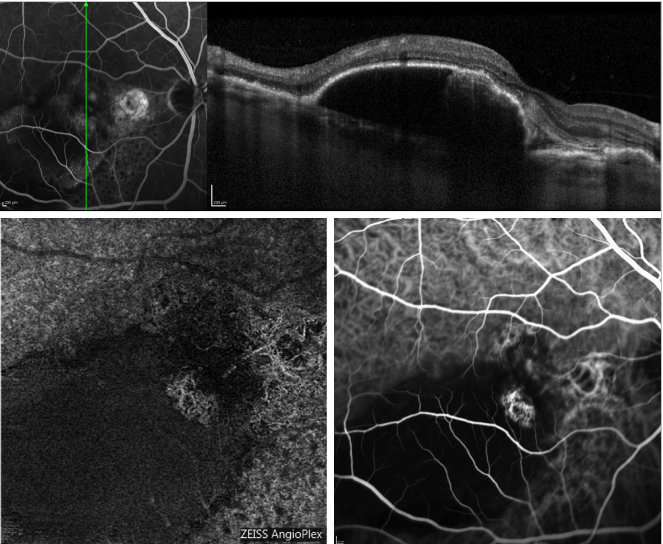
Figure 5 - Serous PED and recurrent PCV (FA, OCT, ICGA and OCT-A). On the top, OCT scan acquired simultaneously with FA; On the bottom, appearance of the polyps profile on OCT-A (left) and ICGA (right).
However, the static nature of this examination and the presence of possible artefacts are important limitations that should be acknowledged.
With regard to serous PED, shadowing due to loss of signal transmission in correspondence of the PED may make it difficult to detect the CNV complex on OCT-A (Figure 6).
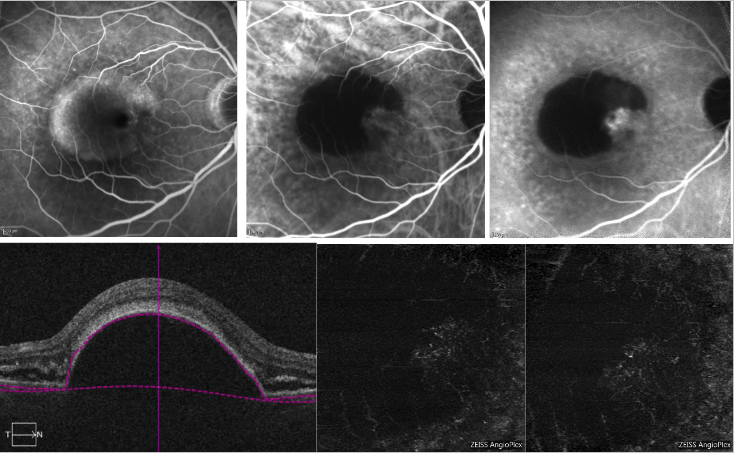
Figure 6 - Vascularized PED and serous PED with Type 1 NV (FA, ICGA and OCT-A) . On the top, FA (left) shows dye pooling due to presence of a serous PED; ICGA (top middle and right) shows a neovascular network on the edge of the PED (notch); On the bottom, OCT-A does not provide a definite imaging of the neovascular net.
Serous PED natural course depends on the presence or not of the neovascular component(34).
In pure serous PED there is generally a slow enlargement of the lesion, with a minimal progression of visual loss over a long period (months or years).
However, many can subsequently develop neovascularization, which make it worse(35).
Natural course in vascularized PED may vary, and it is related to the type of new vessels associated.
The most common acute complication of PED is the tearing of the RPE(36-39).
It usually occurs at the edge of the PED, at the intersection of the detached and attached RPE.
Clinically, RPE tear or rip appears as a well-defined area of bare choroid, contiguous to a darker hyperpigmented rugate area, that corresponds to the mound of the RPE that has torn away(40,41).
The ripped RPE usually rolls towards the CNV, and its propensity to tear can be predicted by the observation of pre-tear characteristics, such as an increase in the size and a modification in the shape, the presence of small holes at the PED margins, the presence of hemorrhages or subretinal fluid, but the most noteworthy aspects are the irregular filling of the PED visible at the FA, height of PED > 580 nm, duration > 4.5 months, hyperreflective radial lines on near reflectance imaging, smaller ratio of vascularized PED and anti-vascular endothelial growth factor (anti-VEGF) therapy(42-46).
RPE tears occurs either spontaneouslyor after a treatment, formally laser photocoagulation, photodynamic therapy and intravitreal injection of steroids or anti-VEGF agents(47- 58).
The exact pathogenesis of RPE tears is poorly understood.
Concerning PEDs’ natural course, it has been hypothesized that tangential shearing forces in the PED can cause the break of the RPE basement membrane at the edge of the detachment; however, it is more likely the result of several variables, where the presence of a CNV plays a major role.
Several causal relationships have been reported for RPE tears occurring after treatment, including the heat generated by photocoagulation, the abrupt increase of intra-PED fluid, a contraction of the associated CNV and the concomitant sudden resolution of the sub RPE fluid.
The combining presence of vitreomacular traction and the deformation of the globe due to the mechanical trauma by the needle have also been reported as causative agents(59).
After RPE rips, the majority of patients complain of a sudden severe visual decrease.
In a small percentage of eyes, where the tear spares the fovea, patients can experience a temporary preservation of good visual function(60).
However, in the long term, the progression of a subretinal scar leads to a severe visual decrease. In the prognosis of serous PED, it must be also considered the high risk of bilateral involvement(61).
Treatment of serous PED, associated or not with CNV, has always been a challenge and so far there are no recommended guidelines for their management.
Pure serous PEDs have been treated in the past with laser grid or scattered photocoagulation, nevertheless with disappointing results(61).
Treatment of serous PED, associated or not with CNV, has always been challenging and there are no recommended guidelines for their management so far. Pure serous PEDs have been treated in the past with laser grid or scattered photocoagulation, with disappointing results(62).
No other approaches have been attempted to treat these lesions.
When a neovascular net is present, treatment of serous PED has been focused on CNV management. However, given that vascularized PEDshave never been included in the major randomized controlled trials, we need to make the treatment decision based on small series published, which are often retrospective and do involve different therapeutic approaches.
Now, in the anti-VEGF therapy era, all the previous employed treatments appear unsatisfactory.
Laser photocoagulation has been widely employed and might still have a limited indication when an ICGA-well defined CNV lies remote to the detached RPE(63).
Verteporfin photodynamic therapy (PDT) alone has been proved to be harmful, increasing the risk of RPE tear, hemorrhages and sudden visual acuity decrease(39,48,49,64) (Figure 7).
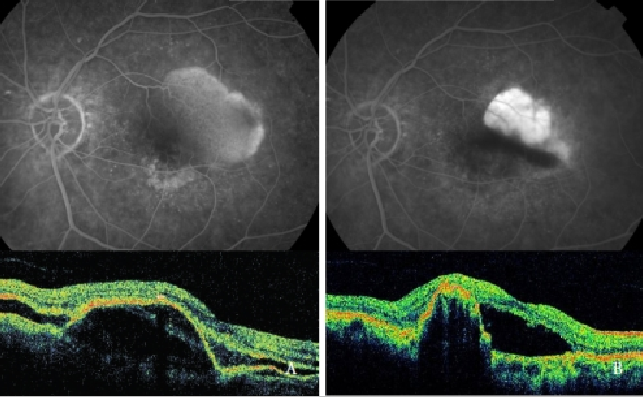
Figure 7 - Vascularized PED with CNV (Type 1 NV) before (left) and after (right) PDT: RPE tear (FA and OCT).
However, PDT combined with intravitreal triamcinolone acetonide injection (IVTA) has been demonstrated as potentially capable of visual acuity stabilization and recurrences reduction(65).
Nevertheless, the high rate complications (cataract and glaucoma) has reduced the use of intravitreal triamcinolone.
After the encouraging results obtained with the anti-VEGF intravitreal therapy in the treatment of occult CNV, the use of anti-VEGF treatment have been extended to vascularized PED with disappointing results(66-69).
Both acute complications and poor anatomical response to the treatment frequently invalidate our attempts to heal the lesion. RPE tears and subretinal hemorrhages have been reported to complicate intravitreal ranibizumab and bevacizumab treatments(51-57).
Moreover, the sub-RPE fluid hardly responds to the anti-VEGF therapy, possibly due to the hydroconductivity changes of Bruch’s membrane(69).
In a retrospective case series of 328 patients treated with bevacizumab, ranibizumab, pegaptanib and PDT+IVTA respectively, after a mean follow-up of 42.4 weeks, the authors reported a significant stabilization of visual acuity in each group, better in the bevacizumab and ranibizumab ones compared to the other two, and an overall RPE tears frequency of 12.5%.
However, they conclude that with these treatments, only a partial regression of the lesions can be obtained, and the risk of RPE tears is not avoided(69).
Another retrospective study(58). reviewed patients outcome of vascularized PED treated with PDT alone, PDT combined with IVTA or intravitreal anti-VEGF injections alone (bevacizumab or ranibizumab) and showed better functional results for the anti-VEGF treatment group.
Moreover, in this series, Type 1 NV with vascularized PED as compared to Type 3 NV, along with a better visual acuity at baseline, showed greater risk of acute RPE tear after treatment(58).
In a recent prospective study(19), treatment of PED associated with subfoveal Type 1 NV with intravitreal ranibizumab injection and with a 3-monthly loading phase and a pro re nata strategy led to only partial results over a 24-month follow-up.
Recently several studies have investigated the efficacy of intravitreal aflibercept therapy of PED in AMD showing good anatomical response with improvement or no significant change in visual acuity(70-73).
Moreover, intravitreal aflibercept has been shown to be a promising treatment in PED resistant to intravitreal ranibizumab treatment(72-74)..
Several studies have identified factors which might influence response of PED to anti-VEGF treatment(75-78).
Dirani et al.(75) showed that better visual improvement was associated with lower baseline visual acuity, presence of subretinal fluid and RAP. Moreover, in their series, PED reduction was associated with higher PED at baseline, predominantly serous PED and use of aflibercept.
Cho et al.(76), in a recent case series, found that lower PED height at baseline, PCV or RAP compared to typical neovascular AMD, serous PED compared to fibrovascular PED, and aflibercept compared to ranibizumab, have an higher chance of PED resolution during anti-VEGF treatment of PEDs.
However, in a recent post hoc analysis of a phase III randomized controlled trial(77), it has been shown that, at 24 months after initiation of anti-VEGF treatment, around half of the patients presenting PED at baseline showed complete resolution of PED regardless of PED status and height at baseline.
Recently Chen et al.(78) outlined the importance of differentiating eyes presenting RAP as they havebetter anatomical and functional outcomes with fewer injections as compared to PED with Type 1 NV.
In the future, new combination therapies and new therapeutic strategies, along with identification of new clinical biomarkers of response to treatment, will help to improve the prognosis of the patients affected by vascularized PED.