Combined Treatment
Combined Treatment
Authors:
Ana Fernandes Fonseca
Instituto Oftalmológico Gama Pinto, Lisbon, Portugal
ALM, Lisbon, Portugal
Mário Guitana, MD
Portuguese Red Cross Hospital, Lisbon, Portugal
Victor Ágoas, MD
Instituto Oftalmológico Gama Pinto, Lisbon, Portugal
Teresa Luísa Quintão, MD
Santa Casa da Misericórdia de Lisboa, Lisbon, Portugal
IRL-Instituto de Retina de Lisboa, Lisnon, Portugal
José Henriques, MD
Instituto Oftalmológico Gama Pinto, Lisbon, Portugal
IRL-Instituto de Retina de Lisboa, Lisnon, Portugal
Updated/reviewed by the authors, July 2017.
1. Introduction
Choroidal neovascularization in AMD has become a serious medical and social problem. One of the reasons for this is ageing of the population. However, a better understanding of this disease and the emergence of new treatment options have been witnessed in recent years.
In clinical practice, combination treatments are necessary when a disease is not well properly controlled with a single therapeutic modality. Using the anti-VEGF gold standard therapy to control exsudative AMD is not enough because it does not structurally change the neovascular membrane, i.e., it does not result in its regression. Combined therapy is the logical step to counteract disease progression mechanisms which are self-supportive once initiated.
We can combine(1,2) anti-VEGF drugs with corticosteroids, verteporfin photodynamic therapy and anti-pericyte agents (targeting vascular endothelial growth factors, angiogenesis-regulating cytokines, proliferating endothelial cells and pericytes, respectively), with the common goal to achieve a synergistic action, with improved outcomes, reduced retreatment frequency and more sustained effects. This additive effect allows patients to be treated with lower doses, entailing added value through increased tolerability and decreased costs.(3).
2. Health economics in AMD treatment: efficacy versus efficiency and the importance of equity
2.1 Seeking efficacy and efficiency – resource saving
The efficacy of a given drug or technique is assessed when comparative studies of visual outcomes are performed.
If the observed outcomes are identical to those observed in previous studies it is concluded that no apparent advantages result from using the technique or drug in question.
However, if the number of treatment sessions decreases, a smaller number of medicine vials is used or patients visit the hospital less frequently, it is concluded that the efficiency of the drug or method is greater.
Drugs and methods that are equivalent in terms of efficacy may vary widely in terms of efficiency. We are thus faced with efficiency gains and better use of resources – more patients are treated with the same budget.
Ophthalmologists have been researching for the more efficacious and efficient treatment regime that allows reducing the therapeutic burden and customize the treatment.(80)
This theoretical improvement in clinical efficiency has the advantage of reducing the number of treatment sessions, with a consequent decrease in the drugs cost used to treat each patient.
Since each patient will make less visits to the hospital, the number of medical, nursing and technical staff hours required will decrease, the same occurring for equipment operation times and time spent at hospital premises.(4)
Different studies refer that the cost of drugs represents the greatest percentage of AMD treatment costs, as opposed to usual health cost distribution, where the largest percentage, of approximately 40%, corresponds to staff costs. In economic studies about the costs of AMD treatment with the ranibizumab protocol, 83% of costs were associated to the drug.(5,6).
2.2 Combined treatments – visual outcomes and efficiency results
It has been proved in a randomized prospective trial that some combination therapies for wet macular degeneration produced better visual results (statistically significant superior efficacy) than monotherapy with anti-VEGF agent.(45,46).
In some other studies, it has been showed that some combination therapies are comparatively effective and cost-effective, with considerably less treatments needed than in monotherapy studies.(7,64)
Calculated costs of 1 year treatment per line of visual acuity were $84 for a regimen of treatment when necessary (PRN) with bevacizumab compared to $766 for the gold standard protocol treatment with ranibizumab. Combined treatment costs varied between $71 and $269.(5,6)
Due to their synergistic effect, combined treatments potentially lead to a decrease in the number of retreatment sessions, as well as sustained long-term visual benefits(1), and better clinical efficiency.
3. Synergistic action and increased treatment effect
How can we explain the fact that a synergistic effect is theoretically achieved by using various mechanisms of action, sometimes more effective than the sum of their separate effects?(Figure 1)
4.3.8 Combination therapy with ICON-1 and ranibizumab
ICON-1 is an anti-Tissue Factor (TF) immunoconjugate protein that binds to pathologic vessels overexpressing TF and acts via a new mechanism of action that can eliminate abnormal CNV as well as inhibit the exudation.
EMERGE(76-77) is a phase 2, randomized, double masked, active control study in the United States that examines the hypothesis that ICON-1 eliminates abnormal CNV as well as inhibits the exudation either alone or in combination with ranibizumab as compared to ranibizumab alone.
A total of 90 patients with treatment naïve (in the study eye) CNV secondary to AMD are being enrolled.
Patients are randomized in a 1:1:1 ratio to receive intravitreal injections of ICON-1 (0.3 mg) as monotherapy (n=30) or in combination with ranibizumab 0.5 mg (n=30) or ranibizumab 0.5 mg monotherapy (n=30). Patients will receive 3 initial monthly injections followed by maximum 3 additional monthly injections based on protocol re-treatment criteria, for a total of 6 months of treatment.
The primary outcomes are mean change from baseline in BCVA letter score and in Central Retinal Thickness (CRT) at 3 months.
Figure 1. Angiogenesis - New blood vessels are formed in response to various physiological and/or pathological stimuli, of which hypoxia is one of the most relevant. Hypoxia activates multiple cellular response cascades, with special emphasis on activation of extracellular matrix metalloproteases and increased synthesis and release of growth factors, including VEGF. The latter acts on membrane receptors, activating intracellular enzyme pathways through intracellular signalling, which results in response amplification. This ultimately leads to cellular proliferation, migration and differentiation, with formation of new blood vessels. Different drug categories act on different stages of the neovascularization process. Joint action on various cascade levels should theoretically lead to an increase in treatment effect and/or a decrease in the effective dose and/or a more prolonged effect.
3.1 Anti-VEGFs
The primary need is to act on the key mechanism of the neovascularization process – VEGF. By acting on this mechanism not only do we inhibit neovascularization but we also act on oedema and inflammatory mechanism, to a certain extent.(9).
Several studies – CATT(10), SECURE(11), HORIZON(12), SEVEN YEAR update in ANCHOR/MARINA(13) – showed that antiangiogenic therapy does not cause neovascular network regression and that mean visual acuity tends to return to baseline when therapy is discontinued.
In fact, anti-VEGF therapy is effective at inhibiting tip cells because these are not protected by pericytes that envelop the already formed neovascular network.(14)
Tip cells and tip cell membrane projections called filopodia, "the fingers that do the walking", "lead the way"(15) aare crucial for neovascularization sprouting. Tip cells produce PDGF-B, a growth factor which stimulates pericyte recruitment to line the neovascular network(16), which means that angiogenesis progression does not depend solely on cell proliferation, but it also requires the formation of a pericytic reinforcement to stabilize the neovascular network. This summarily explains the anti-VEGF resistance described in the literature, because when therapy is discontinued the tip cells become active and grow again(87).
Therefore, additional therapy is needed.
3.2 Synergistic action of corticoids
When steroids are added, a synergistic action is achieved, since steroids act on various levels of the inflammatory process and angiogenesis regulation.
Various mechanisms of action are proposed for steroids in AMD treatment.(79).
It is known that steroids act on local inflammatory mediators, stabilizing blood-retinal barrier function by increasing gap junction density and activity in capillary endothelial cells.
It is thought that triamcinolone decreases VEGF, which is a potent agent in increasing capillary permeability by increasing phosphorylation of proteins involved in intercellular tight-junctions, such as occludin and Zonula Occludens-1 (ZO-1).
These agents also have an anti-inflammatory effect by inhibiting phospholipase A2, an enzyme that metabolizes cell membrane phospholipids to free arachidonic acid, which, in turn, originates thromboxane, leukotrienes and prostaglandins that cause vasodilatation, increased permeability and oedema.
They also have an angiostatic effect by promoting a decrease in extracellular matrix (ECM) turnover through inhibition of plasmin activation.
Plasmin activates matrix collagenases and metalloproteinases (MMP’s) that dissolve the capillary basement membrane and trigger angiogenesis, with endothelial cell differentiation, migration and proliferation (Figure 2).
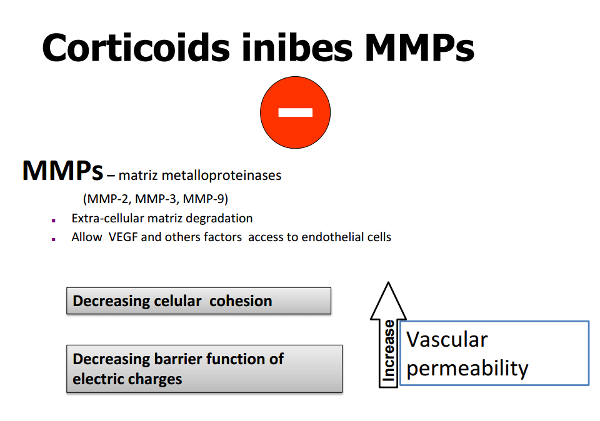
Figure 2. Corticoids inhibit MMP’s (metalloproteinases) activity.
These agents also act on the interaction between ICAM-1 (Intercellular Adhesion Molecule-1) and leukocytes, inhibiting recruitment of the latter, thereby contributing to reduce the inflammatory component.
It is also thought steroids may act on SDF-1 (Stromal-cell Derived Factor-1), inhibiting its action (Figure 3)(8,17).
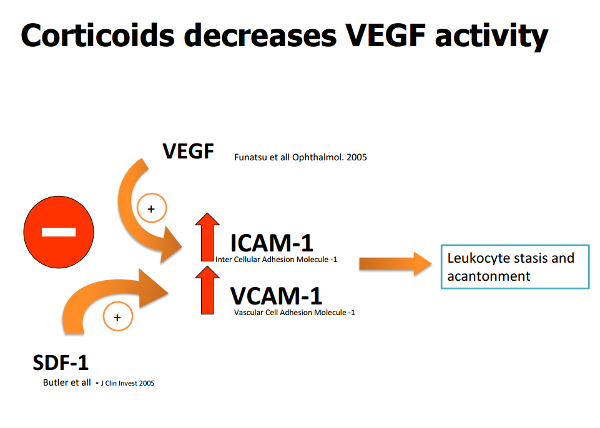
Figure 3. Corticoids decrease VEGF, SDF - 1, ICAM - 1 and VCAM - 1 activity.
Steroids also decrease the expression of Major Histocompatibility Complex Class II (MHC-II) molecules involved in the inflammatory process.(18)
Therefore, we have scientific grounds supporting the combined action of treatment with corticoids (Figure 4).
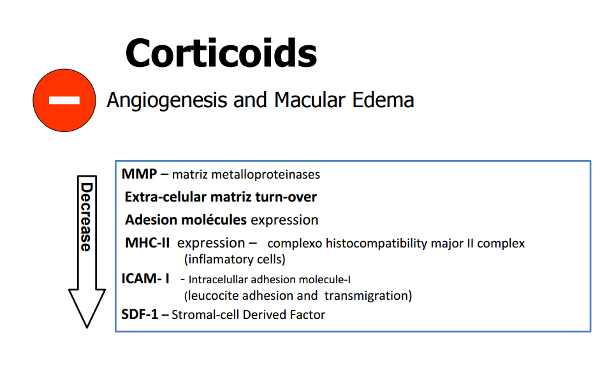
Figure 4. Corticosteroids inhibit angiogenesis and macular edema through multiple mechanisms of action. See text.
3.3 PDGF-B inhibition
Inhibiting PDGF-B facilitates the action of anti-VEGF therapies by inducing pericyte stripping, thereby allowing anatomical regression of the neovascular membrane (see lower section 4.4.1 on Fovista).
3.4 Associated FGF-2 inhibition and PEDF action
It is also possible to inhibit other factors, such as FGF-2, or induce PEDF locally, which has antiangiogenic effects that counteract the angiogenic effect of VEGF.(15-17).
3.5 Acting on the structural level by damaging newly formed blood vessels – PDT
The actions already referred involve blocking or inhibiting neovascularization and inflammatory mediators.
However, it is also possible to act on a structural level, by damaging newly formed blood vessels.
This is achieved through cellular damage and death mediated by free radicals induced by the action of laser on a photosensitizing agent – verteporfin.(18,19).
Standard fluence values of 50-J/cm² or lower fluence values of 25-J/cm² or 12-J/cm² are normally used in combined treatment.
Photodynamic therapy causes vascular occlusion but is associated to an inflammatory response that may be minimized by using corticoids and an anti-VEGF agent.
Both agents may also inhibit the angiogenic stimulus represented by a VEGF rebound effect following occlusion of new blood vessels.(27,78).
Capillary occlusion induced by PDT leads to hypoperfusion of the treated area, a condition that is theoretically worsened by concomitant use of anti-VEGF agents that prevent recapillarization.
This effect has not been shown as negative; on the contrary, it appears that this recapillarization delay promotes neuronal recovery by decreasing oxygen and free radical concentrations.(27,78).
3.6 Other sites of action – associated surgical therapy
We shall not elaborate on this treatment combination, as it will be referred in a chapter dedicated to surgery.
4. Main studies
4.1 Gold standard treatment
The efficacy and safety of combined treatments are evaluated in studies where drug combinations are compared with the gold standard treatment, which, according to the results of the MARINA(28) and ANCHOR(29) studies, consists of twelve consecutive monthly intravitreal injections of an antiangiogenic agent.
Use of this treatment regime led to outcomes of 90% in vision stabilization and approximately 30-40% of significant improvement after one year.
4.2 Combined treatments: double and triple treatments
Most combined treatments that have been used include reduced-fluence PDT associated to an anti-VEGF agent. PDT, antiangiogenic agents and steroids are often used in triple treatments.(31,82)
Combined therapy with PDT and anti-VEGF therapy seems to have a valid role, as suggested by the RADICAL(35) study results.
Dexamethasone seems to be preferable to triamcinolone since there is a decreased risk of ocular tension increase.(31) .
There are no precise guidelines on triple therapy indications, on dosage and type of drugs.
Some combined-treatment studies should be referred for their relevance, albeit in a summarized manner.
4.2.1 SUMMIT Trial programme
This program includes three large randomized clinical trials, DENALI (USA), MONT BLANC (Europe) and EVEREST (Asia), whose objective is to evaluate the efficacy and safety of combining PDT (Visudyne®) and ranibizumab, compared to monotherapy with this antiangiogenic agent, in patients with neovascular AMD and polypoidal disease (EVEREST).
Analysis results from the MONT BLANC(32)
study at twelve months have shown no significant differences between the two groups, although the number of treatments required is slightly lower with the combined treatment.
The DENALI(33) study had a similar result. The overall benefit for patients was a reduced frequency of Lucentis to at least three months during the study.
In the EVEREST(34) study, in the patients with polypoidal vasculopathy, the combined treatment was more effective than monotherapy to promote regression of polypoid formations.
4.2.2 RADICAL Phase 2 study (Reduced Fluence Visudyne-Anti-VEGF-Dexamethasone In Combination for AMD Lesions)
In this study, three Visudyne–Lucentis combination therapies were evaluated against Lucentis monotherapy.
Two-year, phase 2 final 24 months results(35). showed that the combination approach led to significantly fewer visits at which re-treatments were applied. Of the four treatment groups, the triple therapy half-fluence group had the fewest retreatment visits compared with Lucentis monotherapy.
Through 24 months, patients in the triple therapy half-fluence group had a mean of 4.2 retreatment visits compared with 8.9 for patients who received Lucentis monotherapy (P<.001).
More recently, Gallemore et al. conducted a phase II randomized trial and and verified that combination therapy resulted in significantly fewer retreatment visits than a ranibizumab monotherapy regimen at months 12 and 24.(81)
4.2.3 PDEX II study
This was a prospective, multicentric, randomized, non-inferiority study comparing the relative advantages of treatment with PDT in a reduced dose (reduced fluence), dexamethasone and ranibizumab versus monotherapy with ranibizumab.(37)
Trial results were presented at the American Society of Retina Specialists meeting 2008, which compared combination therapy using reduced fluence PDT, intravitreal dexamethasone 500 mcg and intravitreal ranibizumab 0.5 mg, to monthly ranibizumab.
Patients receiving the triple therapy needed fewer treatments than 12 per year.
4.2.4 LuceDex study
This study(36) researched the role of dexamethasone in neovascular AMD treatment.
This is a prospective, randomized clinical trial comparing two treatment groups, one treated with a combination of ranibizumab and dexamethasone and the other undergoing ranibizumab monotherapy.
The LuceDex pilot study suggested a possible benefit of adding intravitreal dexamethasone to treatment of neovascular age-related macular degeneration with intravitreal ranibizumab.
Choroidal neovascular membrane size decreased in Group 1 significantly compared with Group 2 (P < 0.05).
A larger study is needed to further identify and define possible benefits of this combination therapy.
4.2.5 Cave study
In the multicenter CAVE study(38,39), reduced-fluence PDT plus bevacizumab plus triamcinolone therapy was compared with bevacizumab monotherapy, as well as with PDT plus bevacizumab dual therapy. Data from 103 patients were evaluated at one year.
Mean retreatment rates were highest with bevacizumab monotherapy (4.57) and lowest with triple therapy (3.18).
There were no significant between-group differences in mean VA gains. Interestingly, the greatest extension in treatment-free interval was with dual therapy, leading the investigators to conclude that addition of triamcinolone did not produce additional benefit.
4.2.6 Anti-Inflammatory treatment (Dexamethasone implant in combination with ranibizumab)
Six-month trial of 243 patients with CNV secondary to AMD, adjunctive therapy with the dexamethasone implant (Ozurdex) delayed the time to as-needed injections of 500 µg ranibizumab and reduced the need for repeated ranibizumab injections.
DEX Implant delayed the time to as-needed injection of ranibizumaband reduced the need for repeated ranibizumab treatment in patients with CNV secondary to AMD.(40)
4.2.7 Anti-Inflammatory treatment. Pilot Study (Intravitreal ranibizumab and topical bromfenac)
This was a single-site, multiinvestigator, prospective, open-label, interventional, phase II study of patients with new or recurrent exudative/neovascular age-related macular degeneration.
Thirty eyes were enrolled consecutively and were randomized in a ratio of 2:1 to combination therapy with intravitreal ranibizumab and topical bromfenac, and ranibizumab alone.
This pilot study(41) was the first to prospectively identify a biologic signal that may indicate combination therapy with an easily administered well-tolerated eyedrop and ranibizumab is efficacious for the treatment of neovascular age-related macular degeneration.
4.2.8 CABERNET study
The phase 3, multicenter, prospective, randomized CABERNET(42) ((CNV secondary to AMD treated with beta radiation epiretinal therapy) study included a treatment arm of 302 patients receiving strontium-90 epimacular brachytherapy (NeoVista) and two mandatory ranibizumab injections, followed by as-needed ranibizumab injections.
The control arm consisted of 155 patients receiving ranibizumab on a modified PIER protocol with 10 mandatory injections.
The CABERNET study did not achieve its endpoint with a 10% non-inferiority margin.
4.2.9 Combination therapy with low-dose transpupillary thermotherapy and intravitreal ranibizumab
Treatment with low-dose TTT reduced the number or intravitreal injections of ranibizumab over 24 months.
The results suggested that low-dose TTT can serve as an adjuvant in combination with intravitreal ranibizumab for neovascular AMD(43).
4.2.10 ATLANTIC Study
ATLANTIC study (Protocol ECR-AMD-2015-09) is a still running randomized, double-masked, sham-controlled phase 4 study of the efficacy, safety, and tolerability of intravitreal aflibercept monotherapy compared to aflibercept with adjunctive photodynamic therapy in patients with polypoidal choroidal vasculopathy.
4.2.11 VIA study
The objective of this study(7) was to determine whether a combination of a reduced dose of PDT and bevacizumab leads to a decrease in the number of treatment sessions required within a 6-month period, compared to monotherapy with bevacizumab.
This randomized, double-blind, controlled clinical trial revealed that a combination of bevacizumab and 25-J/cm² or 12-J/cm² PDT led to a decrease of approximately 50% in the number of treatment sessions required within a 6-month period.
Favourable outcomes were also observed for visual acuity, although evaluation of this parameter was not the main objective of this study.
Other studies(83-85) confirm the favourable outcome and a consistent reduction of the number of injections, including a study using intravitreal pegaptanib and low-fluence PDT.
4.2.12 PDT combined with vitrectomy and dexamethasone
A combined pharmacosurgical intravitreal procedure was performed 24 to 36 hours after PDT, consisting of 23-gauge core vitrectomy and intravitreal substitution with BSS, dexamethasone and bevacizumab.
The intravitreal retreatment rate was low (13/52) for this safe pharmaco-surgical regimen, corresponding to 25%.(44)
4.2.13 Triple-Combination Therapy and Zeaxanthin
Reports of triple-combination therapy for wet AMD suggest a benefit, as do reports for zeaxanthin.
An interventional comparative study(64) was undertaken to evaluate the efficacy of triple combination therapy with and without zeaxanthin, as well as the economic viability of the therapies.
Cases of 543 consecutive eyes with subfoveal CNV secondary to AMD were reviewed.
All eyes were treated with triple-combination therapy consisting of reduced-fluence PDT, intravitreal bevacizumab and intravitreal dexamethasone. Therapy was repeated as necessary.
One cohort was also given supplementation with 20 mg of oral zeaxanthin (Zx) daily.
The triple-therapy group without Zx received a mean of 2.8 treatment cycles, and 87 percent of subjects had stable or improved vision at 24 months.
In the triple-therapy group with Zx, the mean number of treatment cycles was 2.1, with 83 percent of people having stable or improved vision at 24 months.
Also at 24 months, CNV developed in 12.5 percent of fellow eyes treated with triple therapy alone and in 6.25 percent of eyes undergoing triple therapy with Zx (p=0.03).
Triple therapy was comparatively effective for neovascular AMD, since considerably less treatment was needed than they said has been reported in monotherapy studies.
The addition of oral Zx appeared to further reduce treatment cycles required and risk of CNV development in fellow eyes.
4.3 Other promising forms of combined therapy
4.3.1 FovistaTM (pegpleranib)
In pre-clinical models, combination of anti-PDGF and anti-VEGF successfully induced neovascular regression (fig.5).(21)
Figure 5. Combination of anti-PDGF and anti-VEGF induced angiogenic vessel regression in choroidal neovascular model. Preclinical model of the combined treatment.
Fovista is a 50 kD DNA anti-platelet-derived growth factor aptamer (directed against PDGF-B subunit B) that strongly binds to PDGF-B, and strips pericytes from mature neovascular vessel, leaving them more susceptible to treatment with an anti-VEGF agent.
In dec 2016 Ophthotech completed two large, prospective, randomized, controlled Phase 3 clinical trials of 1248 patients with wet AMD.
The goal of the study - a superiority trial- was to assess the safety and efficacy of a combination of the FovistaTM plus ranibizumab compared with ranibizumab monotherapy in patients with wet AMD.
Ophthotech announced that the "pre-specified primary endpoint of mean change in visual acuity at 12 months was not achieved in its two Phase 3 clinical trials"(88-90).
The study goes on the second year. While these results are disappointing, it remains to be seen if Fovista, in combination with the Anti-VEGF might be able to reduce the number of required injections and visits, or result in a better or more stable vision in the long term.
In the phase 3 trials, the anti-PDGF and anti-VEGF drugs were administered 30 minutes apart. However, in a small investigator-sponsored trial, Pravin Dugel administered the anti-PDGF a day before he injected the anti-VEGF.
This protocol which involved 30 patients with AMD, 27 of whom were treatment-resistant and who had persistent or recurrent fluid and no improvement in visual acuity and demonstrated encouraging results.(92) endpoint with a 10% non-inferiority margin.
Two simultaneous clinical trials have been in progress with the drugs Avastin and Eylea(91), and the complete the results have yet to be released.
4.3.2 FGF-2 inhibition
RPE from CNV patients expresses angiogenic growth factors whose action is partly independent from VEGF.
In a study(51), Sthal concluded that anti-VEGF treatment (bevacizumab) inactivated all RPE-derived VEGF in a 3D collagen matrix culture of RPE isolated from surgically excised CNV-membranes (CNV-RPE) used to stimulate sprouting of endothelial cell (EC) spheroids, but was unable to fully inhibit EC sprouting induced by CNV-RPE. Combined anti-VEGF/anti-FGF treatment inactivated both growth factors and reduced EC sprouting significantly.
In a comparison between the antiangiogenic effect of solitary anti-VEGF antibodies and combination treatment with anti-VEGF and anti-FGF-2 antibodies, greater inhibition was achieved for the latter.
Targeted combined therapy can be better than anti-VEGF monotherapy.
4.3.3 The balancing effect of PEDF and its delivery
In AMD, pigment epithelium-derived factor (PEDF) is significantly lower in RPE cells, the RPE basal lamina, Bruch’s membrane and choroidal stroma. These data suggest the existence of a critical balance between PEDF and VEGF and the hypothesis that PEDF may be able to counteract the angiogenic potential of VEGF.
A decrease in PEDF may disrupt this balance and CNV in AMD(52).
Results from a phase I clinical trial of intravitreal administration of an IE4-deleted adenoviral vector expressing human pigment epithelium-derived factor (AdPEDF.11) suggest that antiangiogenic activity may be sustained for several months after single intravitreal injection of AdPEDF.11(53).
This study provided evidence that adenoviral vector-mediated ocular gene transfer might be a viable approach in the treatment of ocular disorders. However, since the publication of phase I results, there is no evidence of further studies on AdPEDF.11 in the treatment of patients with wet AMD.
4.3.4 Ranibizumab and tyrosine kinase inhibitors
In two cases of recurrent wet AMD in which intravitreal ranibizumab was used in combination with oral sorafenib (a tyrosine kinase inhibitor approved for the treatment of some kidney, live rand thyroid carcinomas) improvements were observed in optical coherence tomography(54), indicating this could also be a promising combination treatment.
Following promising preclinical experiments and a phase 1 trial, X-82 (Tyrogenex) is being investigated in a double-masked, placebo-controlled phase 2 study as an oral treatment for patients with wet AMD.(71)
X-82 is a small-molecule tyrosine kinase inhibitor derived from sunitinib (Sutent, Pfizer), a drug that is approved for the treatment of advanced renal cell carcinoma, gastrointestinal stromal tumors, and pancreatic neuroendocrine tumors. Compared with sunitinib, X-82 has a shorter half-life and accumulates less in tissues, it inhibits VEGF and PDGF receptors without the toxicities associated with sunitinib.
X-82 blocks kinase activity associated with all receptor subtypes for VEGF and PDGF, and its potency against all receptor subtypes is thought to provide targeted inhibition at a relatively low dose with reduced risk of side effects.(72)
Oral X-82 provides dual inhibition of VEGF and PDGF via systemic distribution, which may allow it to efficiently target bilateral disease with fewer injections.
A phase 1 trial showed that treatment-refractory and treatment-naïve patients maintained or improved visual acuity and SD-OCT findings with few or no intravitreal injections, when on oral X-82.(73)
A phase 2 trial of oral X-82 at doses up to 200 mg daily (the APEX Study) is in progress.(74) It is a randomized, double-masked, comparative dose trial with placebo control intended to find the most appropriate dose of X-82 for treatment of wet AMD and to observe its safety and efficacy for a full year.
The trial seeks to enroll 132 patients, and it is running at 25 sites across the United States.
Intravitreal anti-VEGF treatments will be administered as needed, based on SD-OCT evidence of increased macular fluid.
The primary endpoint is visual acuity and secondary endpoints include the number of injections required and anatomic changes on SD-OCT.
4.3.5 DE-120 (anti-VEGF/anti-PDGF agent, Santen)
DE-120 (Santen) is a small molecule dual tyrosine kinase receptor inhibitor for both VEGF and PDGF signaling pathways.
DE-120 belongs to a class of active small molecules that inhibit the activity of receptor tyrosine kinases involved in the angiogenic process.
As a single anti-VEGF/anti-PDGF agent, DE-120 has the potential to provide a synergic antiangiogenic effect and reduce the potential side effects associated with intravitreal injection procedures.
Santen is conducting a phase 2 clinical trial(75) in the United States to evaluate the safety and efficacy of intravitreal DE-120 as monotherapy and along with a single injection of aflibercept (Eylea) in subjects with treatment-naïve active subfoveal CNV secondary to AMD.
4.3.6 Combination therapy of the angiopoietin2 antibody nesvacumab and aflibercept
Regeneron Pharmaceuticals and Bayer developed two separate phase 2 clinical studies to evaluate the combination therapy as a co-formulated single intravitreal injection in patients with wet AMD or diabetic macular edema.
Angiopoietins are a family of vascular growth factors.
Preclinical data(66-68) demonstrated that angiopoietins act together with the VEGF family to promote the formation and maturation of blood and lymphatic vessels in the eye.
Ang2 and VEGF together therefore have the potential to influence the pathological development of new blood vessels and the permeability of blood vessel walls in certain diseases of the eye.
4.3.7 Combination therapy of intravitreal anti-VEGF therapy and topical Squalamine
Squalamine is a small anti-angiogenic drug that acts against the development of aberrant neovascularization by inhibiting multiple growth factors, including VEGF, PDGF and basic fibroblast growth factor (bFGF), wich play a role in angiogenesis and ocular neovascular disease.
The topical formulation of squalamine, called OHR-102, is late stage development for the treatment wet AMD. In the IMPACT studyl(69), a phase 2, prospective, randomized, double-masked, placebo-controlled, multicenter study completed in 2015 in treatment naïve patients with CNV due to AMD, all patients enrolled l(142) received ranibizumab at baseline and randomized 1:1 to topical OHR-102 BID (combination group) or placebo vehicle solution BID (monotherapy group).
Patients were followed monthly for 9 months.
Retreatment with ranibizumab was performed by criteria-based PRN ranibizumab IVT injections monthly to month 9. OHR-102 demonstrated an improvement in visual function when used in combination with an anti-VEGF agent versus anti-VEGF monotherapy.
There were no safety issues identified.
An analysis of visual outcome as a function of lesion characteristics (presence and size of classic and occult CNV) was performedl(70) and demonstrated a strong correlation in Figure 5.
Combination of anti-PDGF and anti-VEGF induced angiogenic vessel regression in choroidal neovascular model.
Preclinical model of the combined treatment. the combination therapy group (p=<0.0001) but not with ranibizumab monotherapy.
Angiographic CNV lesion characteristics were predictive of visual acuity outcomes in combination therapy: baseline occult CNV area showed the highest correlation to visual outcome in combination treatment with IVT anti-VEGF and topical squalamine.
Patients with occult CNV <10 mm2 represented an optimal target population for this combination therapy in this clinical trial.
OHR-102 used in combination with an anti-VEGF agent may provide several potential advantages over other combination therapy approaches currently being investigated in clinical studies.
Based on the good results from the IMPACT study in wet AMD, a phase III program is being initiated.
4.3.8 Combination therapy with ICON-1 and ranibizumab
ICON-1 is an anti-Tissue Factor (TF) immunoconjugate protein that binds to pathologic vessels overexpressing TF and acts via a new mechanism of action that can eliminate abnormal CNV as well as inhibit the exudation.
EMERGE(76-77) is a phase 2, randomized, double masked, active control study in the United States that examines the hypothesis that ICON-1 eliminates abnormal CNV as well as inhibits the exudation either alone or in combination with ranibizumab as compared to ranibizumab alone.
A total of 90 patients with treatment naïve (in the study eye) CNV secondary to AMD are being enrolled.
Patients are randomized in a 1:1:1 ratio to receive intravitreal injections of ICON-1 (0.3 mg) as monotherapy (n=30) or in combination with ranibizumab 0.5 mg (n=30) or ranibizumab 0.5 mg monotherapy (n=30).
Patients will receive 3 initial monthly injections followed by maximum 3 additional monthly injections based on protocol re-treatment criteria, for a total of 6 months of treatment.
The primary outcomes are mean change from baseline in BCVA letter score and in Central Retinal Thickness (CRT) at 3 months.
4.3.9 Combination therapy with aflibercept coformulated with rinucumab (CAPELLA study)
CAPELLA(86) study is an ongoing phase 2 double-masked, randomized, controlled, multiple-dose, regimen-ranging study that has enrolled patients with wet AMD who were treated with aflibercept coformulated with rinucumab, an anti–platelet-derived growth factor (anti-PDGF) receptor beta antibody.
Patients were randomly assigned to one of 3 groups: they received fixed doses every 4 weeks of 2 mg aflibercept monotherapy, 2 mg aflibercept/1 mg rinucumab combination therapy, or 2 mg aflibercept/3 mg rinucumab combination therapy.
At week 12, two of the three treatment groups were rerandomized, resulting in five total dosing groups in the second phase of the study.
This study failed to reach its primary endpoint, according to a company press release. At week 12, the combination therapy did not demonstrate an improvement in BCVA compared with aflibercept monotherapy.
The press release reported that patients in the combination therapy arms showed a 5.8-letter improvement in BCVA, compared with a 7.5-letter improvement in the monotherapy arm.
The company reported that combination therapy showed no benefit over monotherapy on anatomic endpoints including reduction in retinal thickness or resolution of subretinal hyperreflective material.
Ocular adverse events at week 12 were more common in the combination therapy groups (23.5% and 20%) than in the monotherapy group (16%), according to the company.
Researchers will reevaluate data at weeks 28 and 52.
5. The future of combined treatment
Only time will tell us whether double and triple treatments will be the gold standard treatment.
In most combined studies, the number of re-treatments needed was lower than with monotherapy. It is known that some patients respond better to monotherapy, while others respond better to combined treatment; this is very likely due to individual patient and disease characteristics.
Intensive research is currently in course regarding alternative actions on crucial neovascularization cascade steps and mechanisms that trigger this process (signalling), since these have the potential to become alternative strategies for the combined treatment of AMD.
Emerging therapies will be described in a separate chapter.
It shall be referred that the complexity of signalling pathways supports the concept of combined therapy as a way of achieving more adequate control of biological functions in general and neovascularization in particularr(62,63).
6. Practical aspects
Combined therapies have long been used in the treatment of oncological and numerous other systemic diseases.
In neovascular AMD, the objective of combined treatments acting upon different stages of the physiopathological process or the signalling pathway that triggers its mechanisms of action is to achieve synergistic action; therefore, an increased treatment effect and/or a decrease in the number of retreatment sessions required to stabilize vision are to be expected, as well as a more prolonged effect, smaller doses and increased drug tolerance(1).
Treatment of this disease focuses on three main targets(1):
- neovascularization;
- angiogenic process;
- inflammatory cicatricial and exudative process.
In clinical practice, the following therapies are currently used in combined treatment:
- Photodynamic therapy with verteporfin, with standard, low or very low fluence.
- Antiangiogenic agents ranibizumab 0.5mg (Lucentis®), bevacizumab 1.25mg (Avastin®) and VEGF Trap – aflibercept 2 mg (EYLEA®).
- Anti-inflammatory treatment with intravitreal dexamethasone with or without core vitrectomy.
6.1 Particular cases of AMD
Due to their poor response to monotherapy, cases of Retinal Angiomatous Proliferation (RAP) and Polypoid Choroidal Vasculopathy should be primary candidates for anti-VEGF-based combined treatment.
In the Everest Study,verteporfin PDT combined with ranibizumab 0.5 mg or alone was superior to ranibizumab monotherapy in achieving complete regression of polyps in this 6-month study in patients with symptomatic macular polypoidal choroidal vasculopathy.(34).
ATLANTIC study (Protocol ECR-AMD-2015-09), sponsored by AIBILI, Coordinating Centre of EVICR.net, is a still running randomized, double-masked, sham-controlled phase 4 study of the efficacy, safety, and tolerability of intravitreal aflibercept monotherapy compared to aflibercept with adjunctive photodynamic therapy in patients with polypoidal choroidal vasculopathy.
6.2 Improved healthcare and increased equity
If combined treatments are proved to lead to better outcomes (greater VA line gains) or more sustained gains, as referred in most studies, the superior clinical efficacy of this treatment approach will be established.
Although the costs of two or three different treatments need to be considered when calculating combined treatment costs, costs per patient will be reduced if fewer overall resources are used.
This is a more efficient strategy, as well as a principle to follow in health economics: to manage scarce resources so that health investments may benefit more patients, instead of necessarily making expense cuts.
It is also about increasing equity – increasing the number of patients benefiting from treatment(1).
Levels of clinical evidence:
Level I– At least one well-designed study – randomized, controlled studies.
Level II-1– High-quality, non-randomized, controlled studies.
Level II-2– Studies with a control group involving more than one research centre or group.
Level II-3– Studies with no control group; series studies, with or without intervention.
Level III– Opinion of respected authorities, based on clinical experience, descriptive studies or specialised committee reports.
Abbreviations
ACE- angiotensin converting enzyme
AR- angiotensin receptor
ICAM-1- Intercellular Adhesion Molecule-1
SDF-1-CRXR4 Axis– Stromal-Derived Factor-1 and its CRXR-4 receptor
PEDF- Pigment Epithelium-Derived Factor
VEGF- Vascular Endothelial Growth Factor
References - Combined Treatment
References - Combined Treatment
- Schmidt-Erfurth UM, Richard G, Augustin A, Aylward WG, Bandello F, Corcostegui B, Cunha-Vaz J, Gaudric A, Leys A, Schlingemann RO. Guidance for the treatment of neovascular age-related macular degeneration. Acta Ophthalmol Scand. 2007;85(5):486-494.
- Zuluaga MF, Mailhos C, Robinson G, Shima DT, Gurny R, Lange N. Synergies of VEGF inhibition and photodynamic therapy in the treatment of age-related macular degeneration. Invest Ophthalmol Vis Sci. 2007;48(4):1767-1772.
- Markabi S. Combination therapy from the regulatory perspective. Retina. 2009;29(6Suppl):S12-S14.
- Karel I. Moznosti a ekonomické ukazatele lécby exsudativní vekem podmínené makulární degenerace s choroidální neovaskulární membránou. Cesk Slov Oftalmol. 2007;63(5):311-319.
- Smiddy WE. Relative cost of a line of vision in age-related macular degeneration. Ophthalmology. 2007;114(5):847-854.
- Smiddy WE. Economic implications of current age-related macular degeneration treatments. Ophthalmology. 2009;116(3):481-487.
- Dugel PU. Anti-Platelet Derived Growth Factor: Where Do We Stand? Paper presented at: American Academy of Ophthalmology Annual Meeting; November 10, 2012; Chicago.
- Dugel PU. Anti-PDGF Combination Therapy in Neovascular Age-related Macular Degeneration: Results of a Phase 2b Study. RetinaToday March 2013. Available at: http://retinatoday.com/2013/03/anti-pdgf-combination-therapy-in-neovascular-age-related-macular-degeneration-results-of-a-phase-2b-study/ . Accessed April 30, 2017.
- Potter MJ, Claudio CC, Szabo SM. A randomised trial of bevacizumab and reduced light dose photodynamic therapy in age-related macular degeneration: the VIA study. Br J Ophthalmol. 2010;94(2):174-179.
- Olk RJ, Peralta E, Gierhart DL, Brown GC, Brown MM. Triple combination therapy and zeaxanthin for the treatment of neovascular age-related macular degeneration: an interventional comparative study and cost-effectiveness analysis. Int J Retina Vitreous. 2015 Nov;1:22.
- Ambati J, Fowler BJ. Mechanisms of age-related macular degeneration. Neuron. 2012;75(1):26–39.
- Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, Grunwald JE, Toth C, Redford M, Ferris FL 3rd. Comparison of Age-related Macular Degeneration Treatments Trials (CATT) Research Group. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration. Ophthalmology. 2012;119(7):1388-1398.
- Silva R, Axer-Siegel R, Eldem B, Guymer R, Kirchhof B, Papp A, Seres A, Gekkieva M, Nieweg A, Pilz S; SECURE Study Group. The SECURE Study: Long-Term Safety of Ranibizumab 0.5 mg in Neovascular Age-related Macular Degeneration. Ophthalmology. 2013;120(1):130-139.
- Singer MA, Awh CC, Sadda S, Freeman WR, Antoszyk AN, Wong P, Tuomi L. HORIZON: an open-label extension trial of ranibizumab for choroidal neovascularization secondary to age-related macular degeneration. Ophthalmology. 2012;119(6):1175-1183.
- Bhisitkul RB, Rofaghaet S, Boyer DS, Sadda S, Zhang K. Year 7 outcomes for ranibizumab-treated subjects in ANCHOR/MARINA: a multicenter, prospective cohort study. Paper presented at: ARVO Annual Meeting; May 8, 2012; Fort Lauderdale,FL. Invest Ophthalmol Vis Sci. 2012; 53(14):3679.
- 16. Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, Betsholtz C.VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161(6):1163-77.
- De Smet F, Segura I, De Bock K, Hohensinner PJ, Carmeliet P. Mechanisms of Vessel Branching Filopodia on Endothelial Tip Cells Lead the Way. Arterioscler Thromb Vasc Biol. 2009;29:639-649.
- Larrivée B, Freitas C, Suchting S, Brunet I, Eichmann A. Guidance of vascular development: lessons from the nervous system. Circ Res. 2009; 104(4):428-41.
- Wang Y, Wang VM, Chan CC. The role of anti-inflammatory agents in age-related macular degeneration (AMD) treatment. Eye (Lond). 2011 Feb;25(2):127–139.
- Funatsu H, Yamashita H, Sakata K, Noma H, Mimura T, Suzuki M, Eguchi S, Hori S.Vitreous levels of vascular endothelial growth factor and intercellular adhesion molecule 1 are related to diabetic macular edema.Ophthalmology. 2005 May;112(5):806-16.
- Butler JM, Guthrie SM, Koc M, Afzal A, Caballero S, Brooks HL, Mames RN, Segal MS, Grant MB, Scott EW.SDF-1 is both necessary and sufficient to promote proliferative retinopathy. J Clin Invest. 2005 Jan;115(1):86-93.
- Penfold PL, Wong JG, Gyory J, Billson FA. Effects of triamcinolone acetonide on microglial morphology and quantitative expression of MHC-II in exudative age-related macular degeneration. Clin Experiment Ophthalmol. 2001;29(3):188-192.
- Schmidt-Erfurth U, Hasan T. Mechanisms of action of photodynamic therapy with verteporfin for the treatment of age-related macular degeneration. Surv Ophthalmol. 2000 Nov-Dec;45(3):195-214.
- Schmidt-Erfurth U, Kiss C, Sacu S. The role of choroidal hypoperfusion associated with photodynamic therapy in neovascular age-related macular degeneration and the consequences for combination strategies. Prog Retin Eye Res. 2009;28(2):145-154.
- Tatar O, Adam A, Shinoda K, Stalmans P, Eckardt C, Lüke M, Bartz-Schmidt KU, Grisanti S. Expression of VEGF and PEDF in choroidal neovascular membranes following verteporfin photodynamic therapy. Am J Ophthalmol. 2006 Jul;142(1):95-104.
- Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431.
- Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S; ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432-1444.
- QLT announces final results from its radical study evaluating verteporfin PDT (Visudyne®) combination therapy in exudative AMD. Press Release, June 22, 2010. Available at: https://globenewswire.com/news-release/2010/06/22/423627/194795/en/QLT-Announces-Final-Results-From-Its-RADICAL-Study-Evaluating-Verteporfin-PDT-Visudyne-R-Combination-Therapy-in-Exudative-AMD.html . Accessed April 30, 2017.
- Augustin AJ, Puls S, Offermann I. Triple therapy for choroidal neovascularization due to age-related macular degeneration: verteporfin PDT, bevacizumab, and dexamethasone. Retina. 2007;27:133-140.
- Larsen M, Schmidt-Erfurth U, Lanzetta P, Wolf S, Simader C, Tokaji E, Pilz S, Weisberger A; MONT BLANC Study Group. Verteporfin plus ranibizumab for choroidal neovascularization in age-related macular degeneration: twelve-month MONT BLANC study results.Ophthalmology. 2012 May;119(5):992-1000.
- 12-Months results from Denali Study evaluating verteporfin PDT (Visudyne®) combination therapy QLT news release, June 15, 2010. Available at: https://globenewswire.com/news-release/2010/06/15/423254/194371/en/12-Month-Results-From-DENALI-Study-Evaluating-verteporfin-PDT-Visudyne-R-Combination-Therapy.html . Accessed April 30, 2017.
- Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y, Kim H, Lai TY, Pilz S, Ruamviboonsuk P, Tokaji E, Weisberger A, Lim TH. THE EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina. 2012 Sep;32(8):1453-64.
- Triple Therapy - PDT Plus IVD and Intravitreal Ranibizumab Versus Lucentis Monotherapy to Treat Age-Related Macular Degeneration (PDEX). https://clinicaltrials.gov/ct2/show/NCT00390208?term=NCT00390208&rank=1 . Accessed April 30, 2017.
- Ranchod TM, Ray SK, Daniels SA, Leong CJ, Ting TD, Verne AZ. LUCEDEX: A Prospective Study Comparing Ranibizumab plus Dexamethasone Combination Therapy Versus Ranibizumab Monotherapy for Neovascular Age-Related Macular Degeneration. Retina. 2013 Sep;33(8):1600-4.
- Buchholz P, Buchholz A, Kirchhof J, Augustin A. Combination Therapies for AMD: Latest Developments. Retinal Physician. 2010 Mar. Available at: http://www.retinalphysician.com/issues/2010/march-2010/combination-therapies-for-wet-amd-latest-developm . Accessed April 30, 2017.
- Sheidow T, Kertes PJ et al. The role of combination therapy in AMD: the Canadian study of Avastin and Visudyne in exudative AMD (CAVE STUDY), paper presented at Retina Congress 2009;sept 30-Oct. 4, 2009; New York, NY.
- Loewenstein A, Kuppermann BD, Weinberger D, Goldstein M, Singer M, Liu CC, Lou J, Li XY, Whitcup SM. Safety and Efficacy of OZURDEXTM (Dexamethasone Intravitreal Implant) as Adjunctive Therapy to Lucentis® in Patients With Choroidal Neovascularization (CNV) Secondary to Age-Related Macular Degeneration (AMD). Invest Ophthalmol Vis Sci. 2010; 51(13):1255.
- Flaxel C, Schain MB, Hamon SC, Francis PJ. Prospective randomized controlled trial of combination ranibizumab (Lucentis) and bromfenac (Xibrom) for neovascular age-related macular degeneration: a pilot study. Retina. 2012 Mar;32(3):417-23.
- Dugel P. Angiogenesis, Exudation, and Degeneration 2012 Meeting in Miami on Februay 4th. The 2-year results of the CABERNET study.
- Söderberg AC, Algvere PV, Hengstler JC, Söderberg P, Seregard S, Kvanta A. Combination therapy with low-dose transpupillary thermotherapy and intravitreal ranibizumab for neovascular age-related macular degeneration: a 24-month prospectiverandomised clinical study. Br J Ophthalmol. 2012;96(5):714-8
- Koch F, Scholtz S, Singh P, Koss MJ. Kombinierte intravitreale Therapie zur Behandlung der altersbedingten Makuladegeneration. Klin Monbl Augenheilkd. 2008;225(12):1003-1008.
- Jo N, Mailhos C, Ju M, Cheung E, Nishijima K, Robinson GS, Adamis AP, and Shima DT. Inhibition of platelet-derived growth factor B signaling enhances the efficacy of anti-vascular endothelial growth factor therapy in multiple models of ocular neovascularization. Am J Pathol. 2006;168(6):2036-2053.
- Ciulla TA, Jaffe GJ, Patel S. Assessment of Retinal Pigment Epithelium (RPE) Atrophy in a Phase 2b Study of a Platelet Derived Growth Factor inhibitor (Fovista®), in combination with a Vascular Endothelial Growth Factor inhibitor (Ranibizumab) for Neovascular Age-Related Macular Degeneration (NAMD). Invest Ophthalmol Vis Sci. 2016; 57(12):4418.
- Stahl A, Paschek L, Martin G, Feltgen N, Hansen LL, Agostini HT. Combinatory inhibition of VEGF and FGF2 is superior to solitary VEGF inhibition in an in vitro model of RPE-induced angiogenesis. Graefes Arch Clin Exp Ophthalmol. 2009;247(6):767-773.
- Bhutto IA, McLeod DS, Hasegawa T, Kim SY, Merges C, Tong P, Lutty GA. Pigment epithelium-derived factor (PEDF) and vascular endothelial growth factor (VEGF) in aged human choroid and eyes with age-related macular degeneration. Exp Eye Res. 2006; 82(1):99-110.
- Campochiaro PA, Nguyen QD, Shah SM, Klein ML, Holz E, Frank RN, Saperstein DA, Gupta A, Stout JT, Macko J, DiBartolomeo R, Wei LL. Adenoviral vector-delivered pigment epithelium-derived factor for neovascular age-related macular degeneration: results of a phase I clinical trial. Hum Gene Ther. 2006;17(2):167-176.
- Diago T, Pulido JS, Molina JR, Collett LC, Link TP, Ryan EH Jr. Ranibizumab combined with low-dose sorafenib for exudative age-related macular degeneration. Mayo Clin Proc. 2008;83(2):231-234.
- Chaudhry N. Oral VEGF receptor/PDGF receptor inhibitor X-82. Paper presented at: the American Academy of Ophthalmology Annual Meeting; November 14-17, 2015; Las Vegas, NV.
- Jackson T. A phase 1 study of oral tyrosine kinase inhibitor (X-82) in previously treated wet age-related macular degeneration. Paper presented at: 15th Annual Euretina Congress; September 17-20, 2015; Nice, France.
- Jackson T. A phase 1 study of oral tyrosine kinase inhibitor (X-82) in previously treated wet age-related macular degeneration. Paper presented at: 15th Annual Euretina Congress; September 17-20, 2015; Nice, France.
- X-82 to Treat Age-related Macular Degeneration. https://clinicaltrials.gov/ct2/show/NCT02348359. Accessed November 28, 2016.
- A Safety and Efficacy Study of DE-120 Injectable Solution for Age-related Macular Degeneration (VAPOR1). https://clinicaltrials.gov/ct2/show/NCT02401945. Accessed November 28, 2016.
- Hanahan D. Signaling vascular morphogenesis and maintenance. Science. 1997;277:48–50.
- Maisonpierre PC, Suri C, Jones PF, Bartunkova S, Wiegand SJ, Radziejewski C, Compton D, McClain J, Aldrich TH, Papadopoulos N, Daly TJ, Davis S, Sato TN, Yancopoulos GD. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277:55–60.
- Bergers G, Song S. The role of pericytes in blood-vessel formation and maintenance. Neuro Oncol. 2005 Oct;7(4):452–464.
- Slakter JS, Ciulla TA, et al. Final Results from a Phase 2 study of Squalamine Lactate Ophthalmic Solution 0.2% (OHR-102) in the treatment of neovascular age-related macular degeneration (AMD). Invest Ophthalmol Vis Sci. 2015;56(7):4805.
- Brown D, Ingerman A, Shearn SP, Slakter JS. CNV lesion characteristics as a predictor of visual outcome in wet AMD patients receiving combination therapy of intravitreal anti-VEGF therapy and topical Squalamine lactate ophthalmic solution. Invest Ophthalmol Vis Sci. 2016; 57(12):4419.
- A Phase 2 Study (EMERGE) Evaluating Repeated Intravitreal Administration of ICON-1 in Patients With Choroidal Neovascularization (CNV) Secondary to Age-related Macular Degeneration (AMD). https://clinicaltrials.gov/ct2/show/NCT02358889. Accessed November 28, 2016.
- Christmas NJ. A Phase 2 Study (EMERGE) Evaluating Repeated Intravitreal Administration of ICON-1 in Patients With Choroidal Neovascularization (CNV) Secondary to Age-related Macular Degeneration (AMD). Invest Ophthalmol Vis Sci. 2016; 57(12):4434.
- Kaiser PK. Strategies for inhibiting vascular endothelial growth factor. Retina. 2009;29(6Suppl):S15-S17.
- Geltzer A, Turalba A, Vedula SS.Surgical implantation of steroids with antiangiogenic characteristics for treating neovascular age-related macular degeneration. Cochrane Database Syst Rev. 2007 Oct;(4):CD005022.